AD04
For Alcohol Use Disorder (AUD)
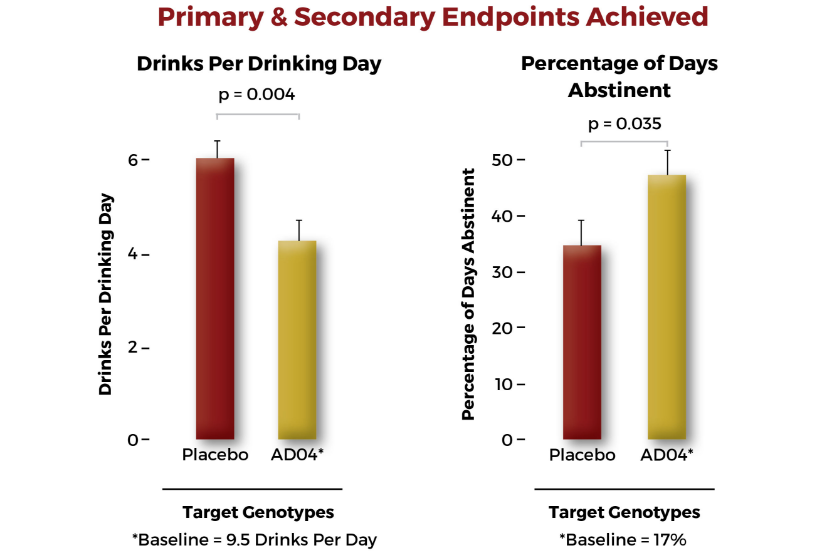
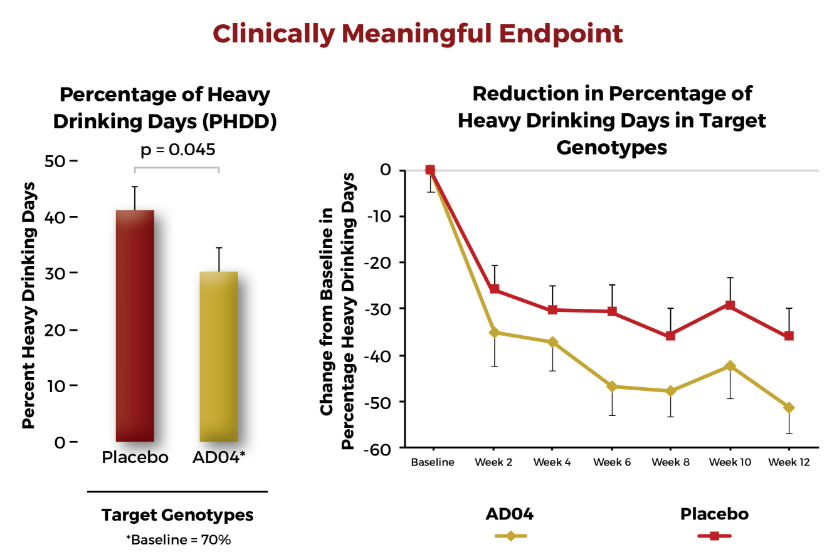
AUD is a disease effecting greater than 35 million people in the US alone. For about 33% of this group there is a specific genetic component which Adial has identified and developed a genetic biomarker test which indicates its lead compound AD04 should be highly effective in controlling cravings and heavy and binge drinking, and support abstinence — going straight to the heart and AUD.
AD04
The Company’s lead compound AD04 (“AD04”) is a genetically targeted therapeutic agent for the treatment of Alcohol Use Disorder (AUD) and is currently being investigated in a Phase 3 clinical trial for the potential treatment of AUD in subjects with certain target genotypes, which are to be identified using the Company’s proprietary companion diagnostic genetic test. Adial possesses a world-wide, exclusive license from the University of Virginia Patent Foundation to commercialize AD04, subject to FDA approval of the product, based upon three (3) separate patents and patent application families, with patents filed and issued in over 40 jurisdictions, including 3 issued patents in the U.S. AD04 has been used in several investigator-sponsored trials and we possess or have rights to use toxicology, pharmacokinetic and other preclinical and clinical data that supports a Phase 3 clinical trial.
AUD is a potentially multi-billion-dollar market with limited competition & unmet need (accounts for ~5.3% of deaths worldwide and ~5.1% of disease worldwide)
The Lancet reports that alcohol is the number one cause of death in the U.S. & globally among both men and women ages 15 to 49 years
Differentiated product
- Designed to reduce drinking levels (believed through reduction of craving)
- Potential to facilitate abstinence
- Limited side effects to date
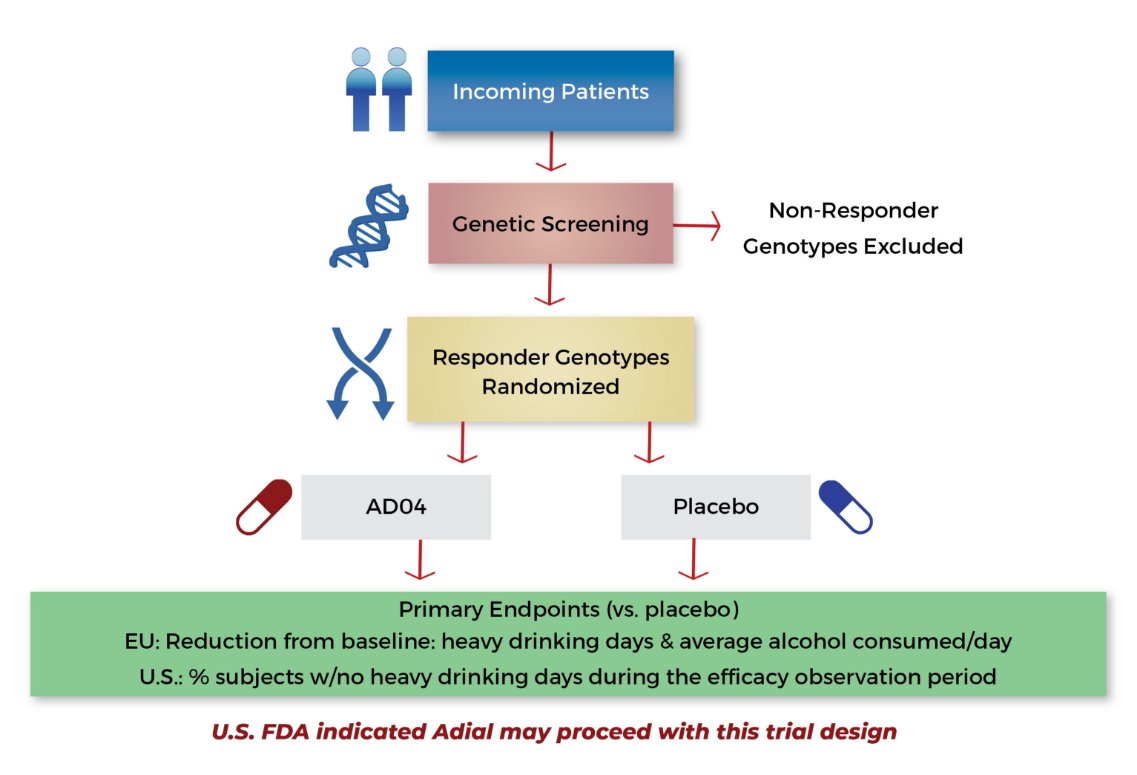
- Companion genetic bio-marker (Genetic test identifies the 33% of patients expected to respond to AD04)

Genetic Test Changes the Conversation
Makes a different conversation with Physician possible
“First step is a test to see if you have a genetic cause for AUD”
Gets Patient buy in
“Treatable Genetic-based disease,” rather than a personal weakness or character flaw.
Expected to increase, fill rate, and compliance,
$35 million have AUD in the USA alone, Today about 8% seek treatment, with this new conversation we expect that number to increase.

AD04 is Designed to Meet the Market Need and Allow Management of Heavy Drinking
New Method of Action (MOA)
for treating AUD
Designed to reduce craving in order to effectively curb alcohol intake
Reduction of heavy drinking target indication
Ends need for abstinence, a major hurdle in starting & continuing pharmacologic therapy
Good safety profile, high tolerability
Brings 20+ year record of acute clinical use with positive safety and tolerability profile
Lowers the stigma of AUD and empowers the patient
Takes treatment from detox clinics & group therapy- realizes patients’ desire of reduced drinking
Oral daily dosing (twice-a-day now,
once-a-day expected)
Maximal patient compliance, ease of use & increased effect
Genetic Tests for Precision Medicine
Companion genetic biomarker test identifies 33% of patients likely to benefit from AD04
AD04 Expected Unique Profile
Compared to Currently Approved Products
Key expected unique selling points drive AD04 differentiation—Expected to meet patient needs
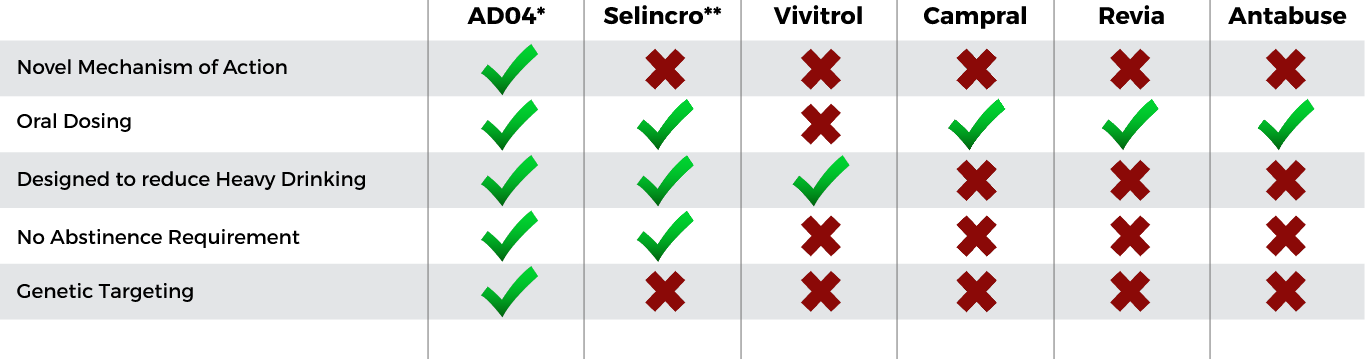
Novel Mechanism of Action for Treating AUD
Studies suggest that blockade of serotonin-3 receptors will influence the dopamine reward system activated by alcohol, decreasing dopamine release and attenuating craving for alcohol
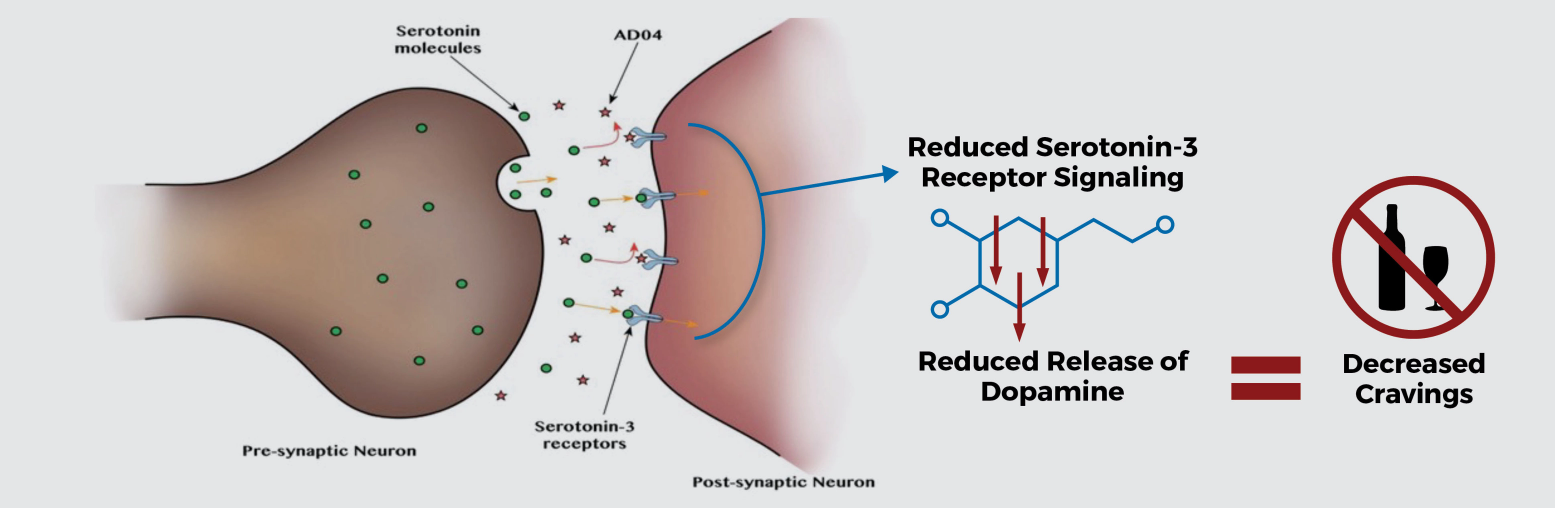
AD04 believed to interfere with the dopamine reward system and lead to reduced alcohol intake
AD04 — Clinical Development Strategy
Run the Phase 3 clinical trials in series, continuing with success
Trial targeting EMA endpoint of reduction of heavy drinking (294 patients)
Data in 2022
Key Dates
Expand trial size (580 patients); powers the study for the U.S. required endpoint*
Data in 2023
Key Dates
Market Launch in 2024
Key Dates
Intellectual Property
Protection through at least 2032
Patents expected to prohibit competitors from bringing ondansetron to market for AUD at any dose and also at the AD04 dose
- 3 patent families under prosecution
- Licensed patents issued in >40 countries, including U.S., Europe & Eurasia
- Includes obesity, drug addiction, smoking, anxiety and related disorders
- Use of ultra-low dose ondansetron (0.33 mg/tab.) pursuant to AD04’s proposed label
- Use of ondansetron to treat any of the four genotypes in the panel
- Potential competitors should be unable to modify the genetic panel without expensive and long clinical trials
AD04 — Commercialization Strategy
Launch commercially with niche sales forces, expanding with success
Commercialization Plan
- Specialty sales force
- Core markets and countries
- Focusing on the top 10k targets in the U.S.
- Focusing on the top 3k targets in the EU5
- Modest sales goals
- General practitioner sales force to saturate market
- Direct to consumer marketing in U.S.
- Expansion beyond core countries
- Blockbuster potential